What are Clinical Trials?
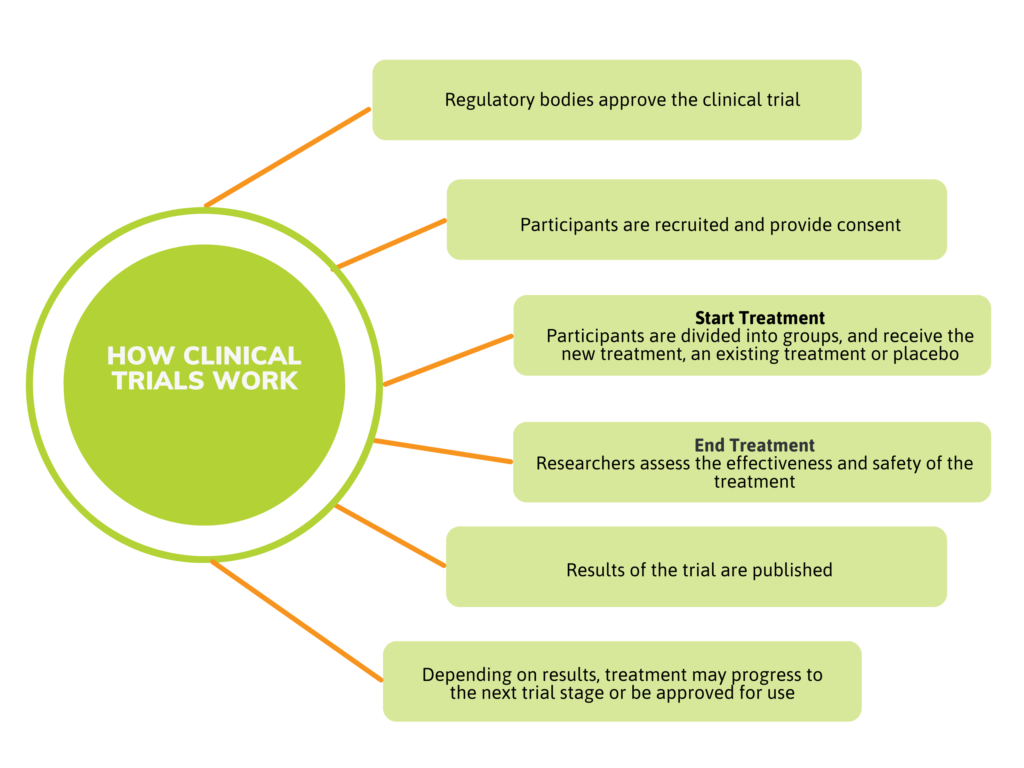
A clinical trial or study is a carefully controlled way to research the effectiveness and safety of new treatments. By the time a treatment is ready for clinical trials, it has already undergone exhaustive testing in the laboratory and in studies with animals.
Clinical trials are conducted under the close supervision of doctors and other research professionals, and have been vetted by Health Canada. The trial is also reviewed by a Research Ethics Board (REB), an independent group of research professionals. The REB ensures that the trial meets the highest ethical standards and is conducted safely.
A common myth is that clinical trial volunteers are guinea pigs, or that clinical trials are used as a last resort. This is not true. If your doctor has recommended a particular clinical trial to you, then it may be the best option for your particular situation, or stage or type of cancer.
If you would like to participate in a clinical trial or learn more about whether a specific trial would be right for you, you should speak with your healthcare team about the benefits and risks.